Much fanfare heralds a new era of AI in precision medicine. LABS is excited to ride the crest of this wave! Several stumbling blocks - reproducibility, disease heterogeneity, and multi-scale modeling - need to be combatted toward future clinical transition.
Multi-organ science provides a holistic way of capturing disease pathogenesis by providing rich data. We know that no organ system operates in isolation. Therefore, more refined disease modeling may offer great potential for precision medicine.
* Wen, J., Tian, Y., (2024) The Genetic Architecture of Biological Age in Nine Human Organ Systems. Nature Aging. Link
* Wen, J., Zhao, B., (2024) The Genetic Architecture of Multimodal Human Brain Age. Nature Communication. Link
* Wen, J., Nasrallah, I., Abdulkadir, A., (2023). Genomic loci influence patterns of structural covariance in the human brain. PNAS. Link
* Wen, J., (2025) Neuroimaging endophenotypes reveal underlying mechanisms and genetic factors contributing to progression and development of four brain disorders. Nature Biomedical Engineering. Link
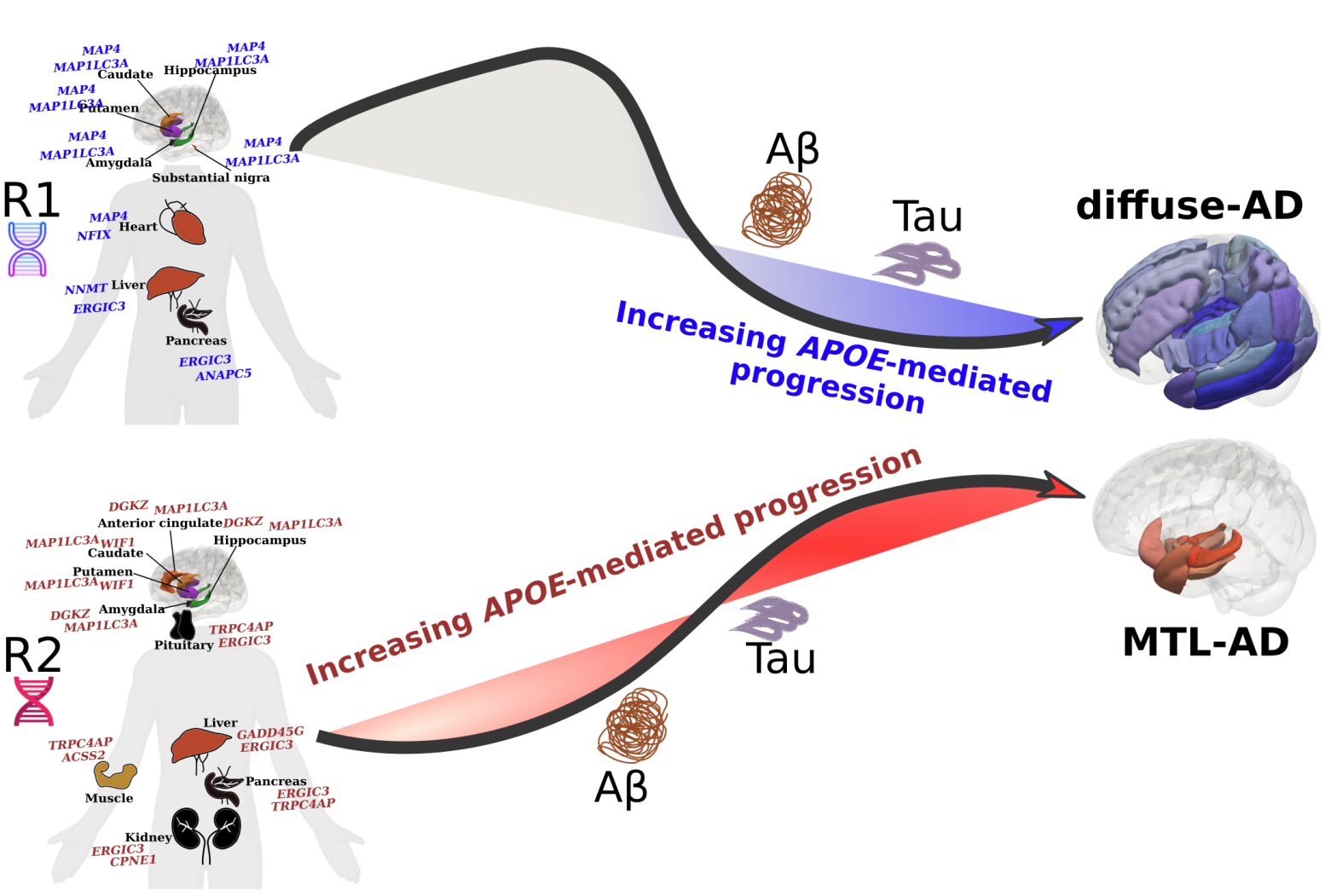
AI-derived endophenotypes are closer to underlying etiology and genetics, thereby providing the potential to model disease heterogeneity. It is well-known that the disease pathogenesis of brain diseases, e.g., AD, is multifaceted. Therefore, modeling disease effects using solely neuroimaging data may be insufficient! This is where other sources of biomedical data may come into play to shed new insights!
* Wen, J., Nasrallah, I., Abdulkadir, A., (2023). Genomic loci influence patterns of structural covariance in the human brain. PNAS. Link
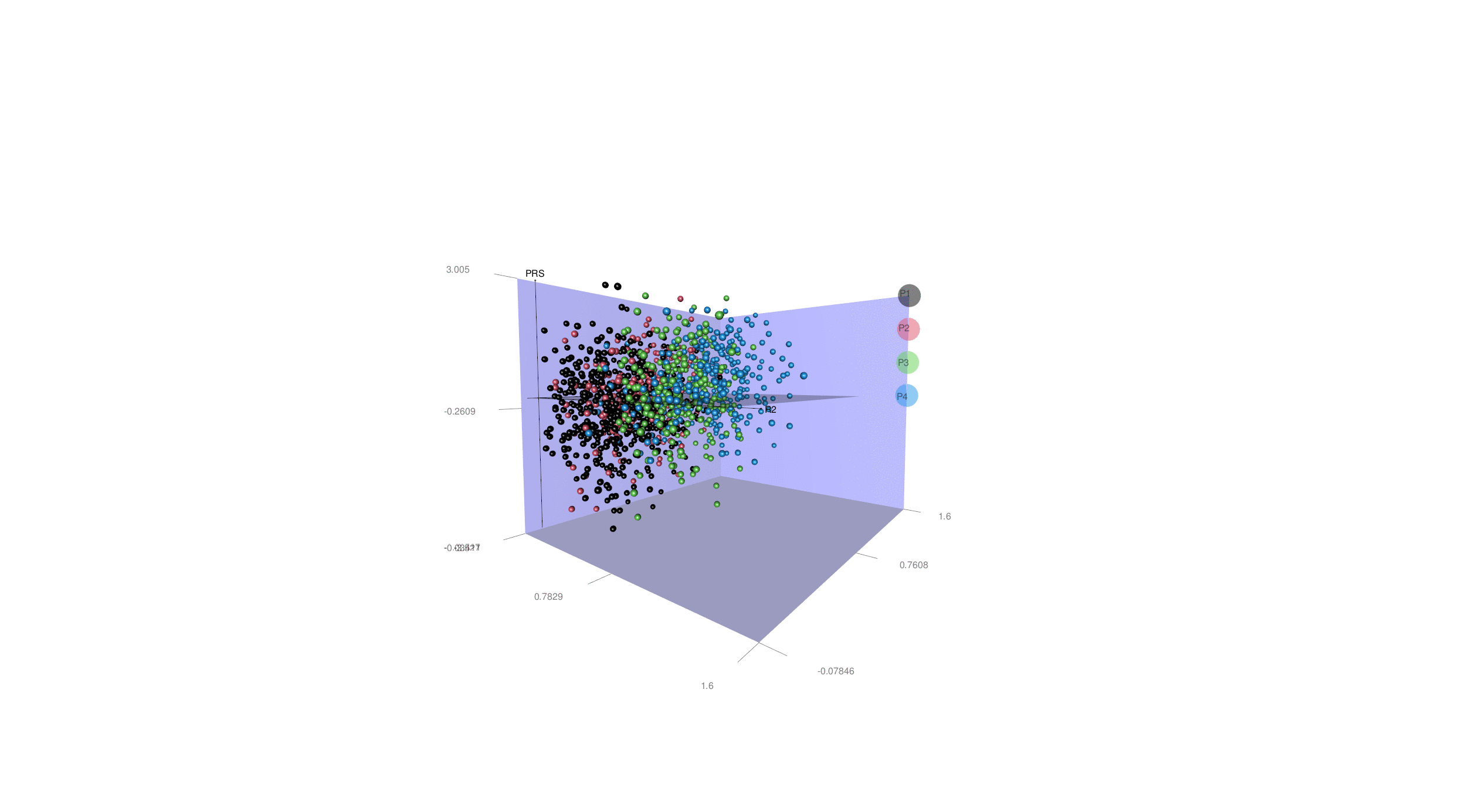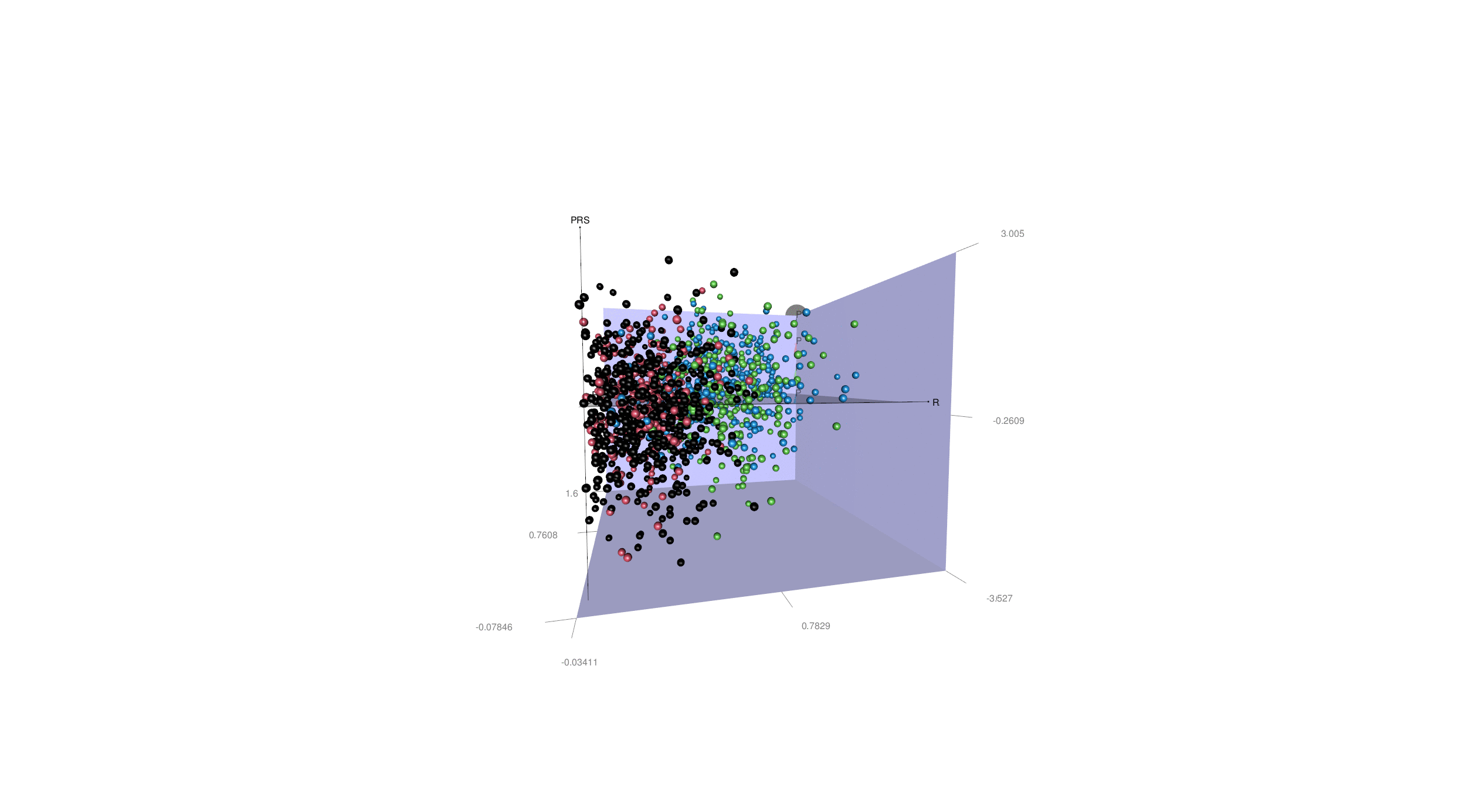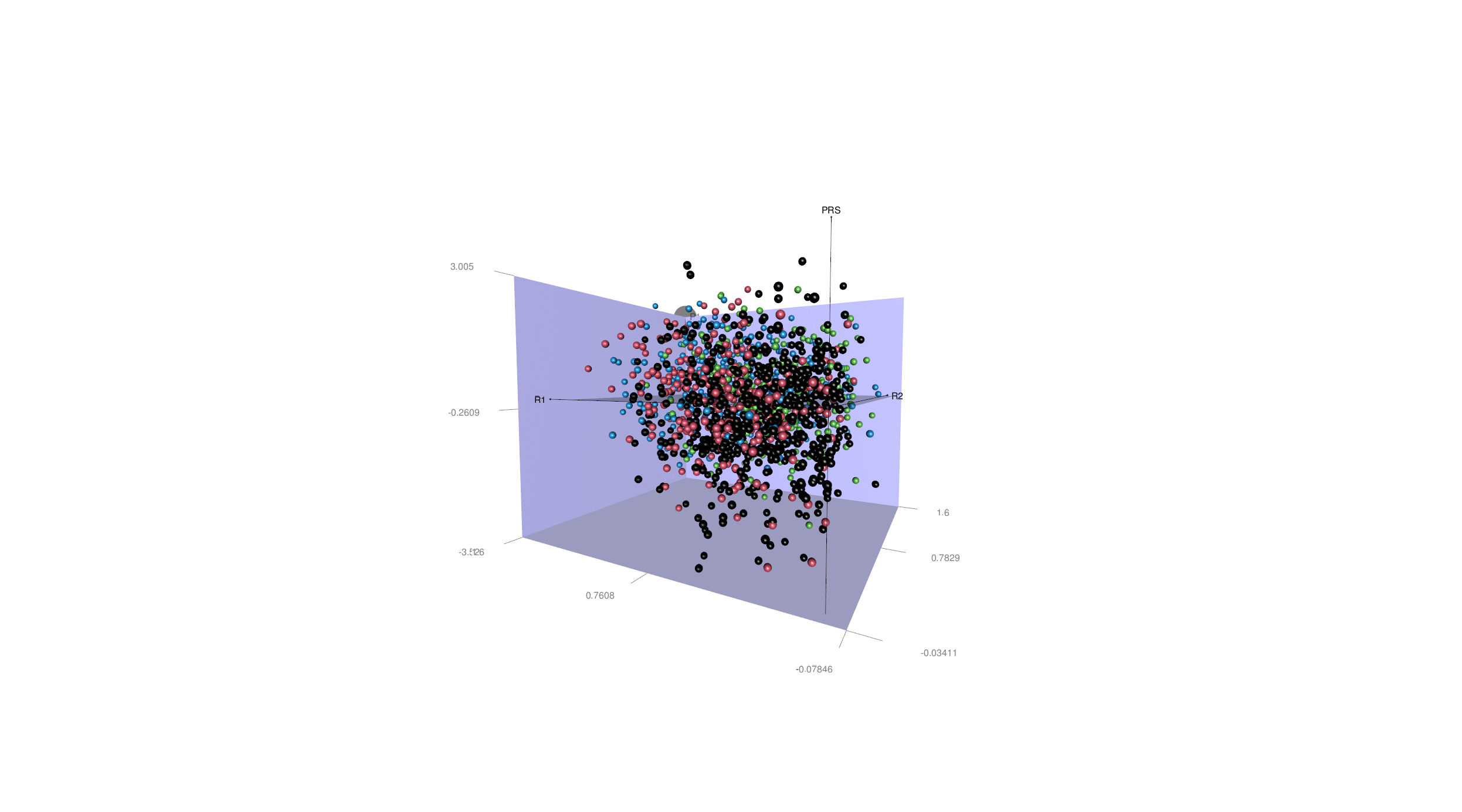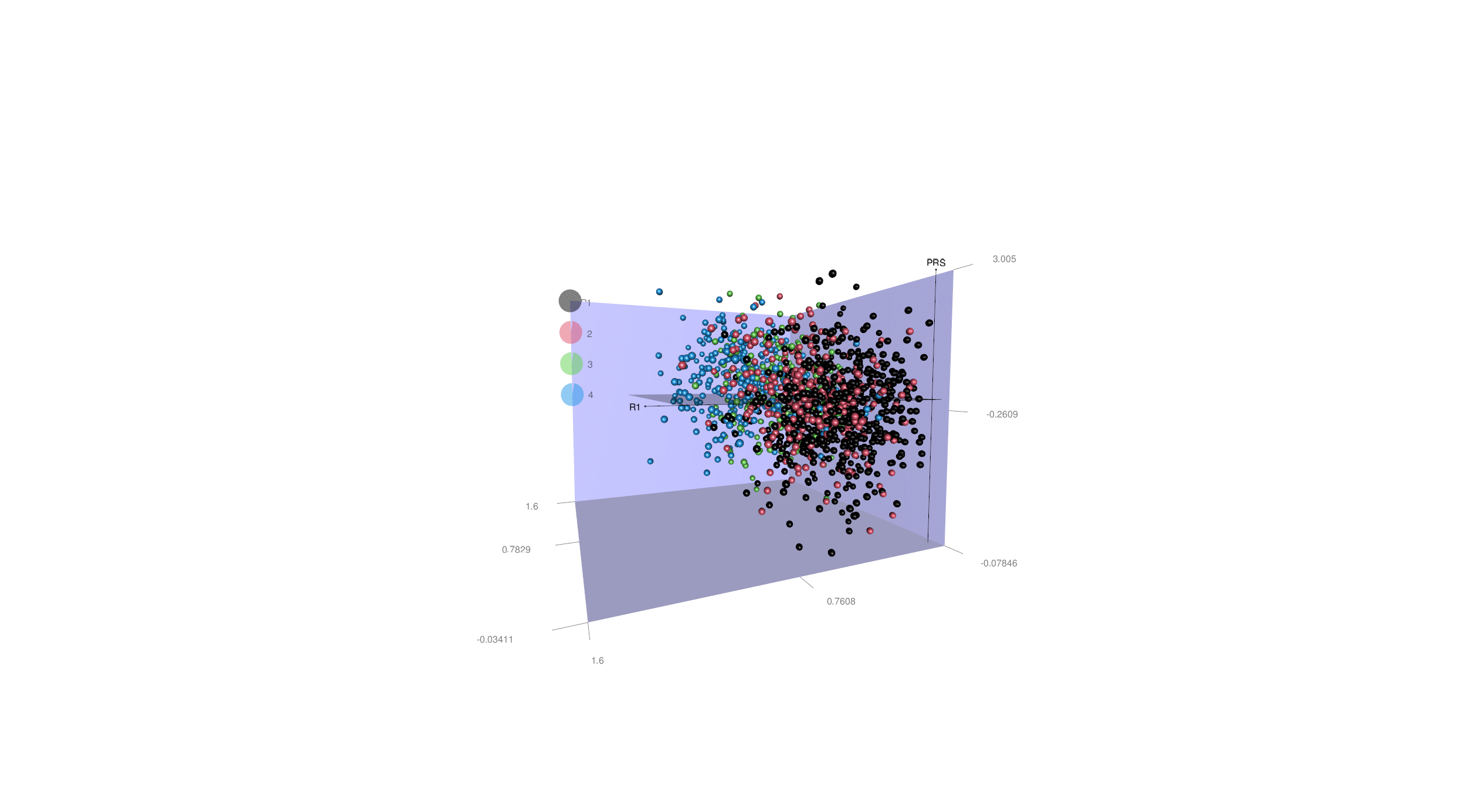
More generally, disease heterogeneity is another critical challenge to paving the road for precision medicine. We can leverage advanced ML & DL techniques to dissect the neuroanatomical heterogeneity of various brain diseases, including Alzheimer's disease (AD), schizophrenia, major depressive disorder, and autism spectral disorder. We recapitulate this heterogeneity at the individual level and place the patients in an N-dimensional representation, indicating the individualized susceptibility to a specific disease. For illustration purposes, in the animation here, we display this heterogeneity in all AD patients with two distinct dimensions (R1 & R2) and four subtypes (A1, A2, A3 & A4), as well as the PRS-AD.
* Wen, J., Fu, C.H., Tosun, D., (2023). Characterizing Heterogeneity in Neuroimaging, Cognition, Clinical Symptoms, and Genetics Among Patients With Late-Life Depression. JAMA psychiatry, 79(5), pp.464-474. Link
* Hwang, G., Wen, J. (co-first), Sotardi, S., (2023) Three Neuroanatomical Endophenotypes of Autism Spectrum Disorder and Their Partially Overlapping Characteristics with Schizophrenia. JAMA Psychiatry. Link
* Wen, J., Varol, E., Sotiras, A., (2022). Multi-scale semi-supervised clustering of brain images: Deriving disease subtypes. Medical Image Analysis, 75, p.102304. Link
* Nasrallah, IM., Abdulkadir, A., Wen, J., (2024). Genetic and Clinical Correlates of AI-Based Brain Aging Patterns in Cognitively Unimpaired Individuals. JAMA Pyschiatry. Link
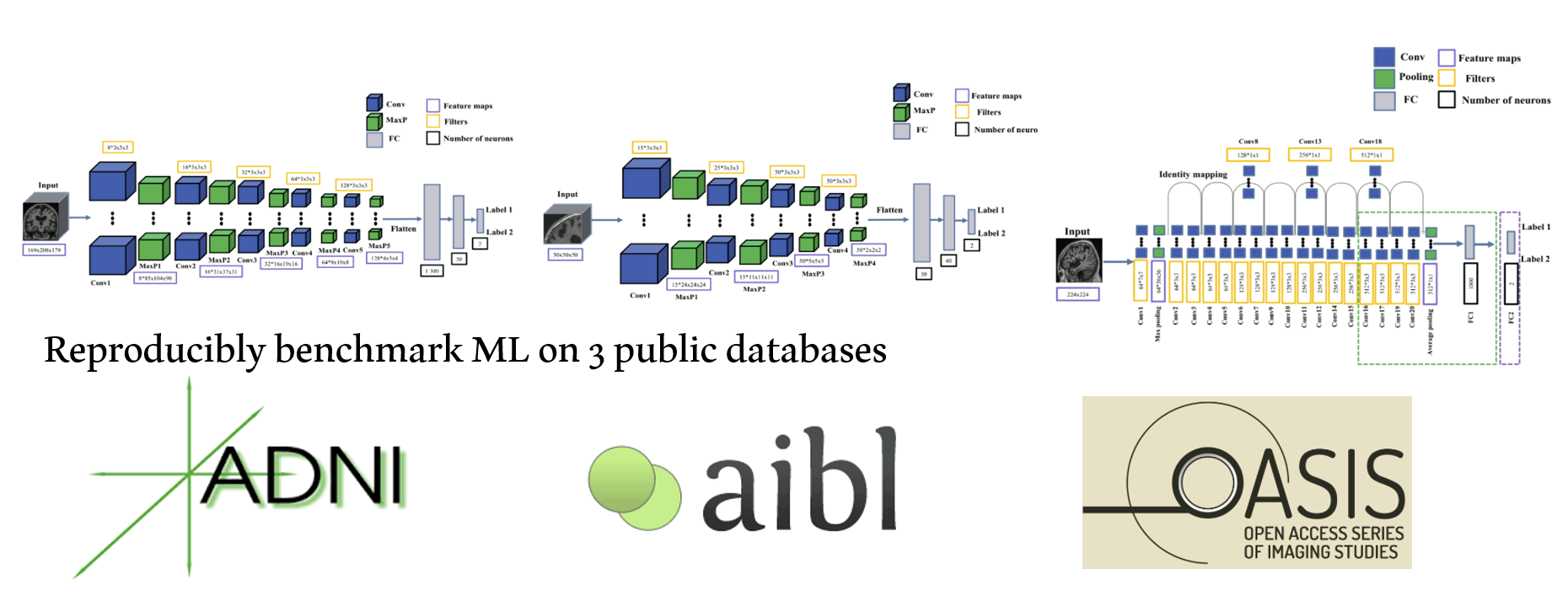
We proposed an open-source framework to objectively and reproducibly evaluate the performance of AD classification using ML (e.g., SVM, random forest), DL (e.g., CNN), and neuroimaging data (T1w MRI, diffusion MRI, and PET). We critically identified one major issue - data leakage - in the field that can hamper the clinical translation of these ML models. What's more, we also demonstrated that a simple linear SVM leads to comparable performance compared to a CNN. All code and results are publicly available.
* Wen, J., Thibeau-Sutre, E., Diaz-Melo, M., (2020). Convolutional neural networks for classification of Alzheimer's disease: Overview and reproducible evaluation. Medical image analysis, 63, p.101694. Link
* Wen, J., Samper-González, J., Bottani, S., (2021). Reproducible evaluation of diffusion MRI features for automatic classification of patients with Alzheimer’s disease. Neuroinformatics, 19(1), pp.57-78. Link